When we go to the doctor for a major surgical procedure such as a total knee replacement, we assume our doctor will use the latest devices and techniques. However, we also assume any equipment used will have been subject to clinical trials and approved by the United States Food and Drug Administration (FDA).
 Unfortunately, according to a recent news article from the New York Times, things don’t always work out so well. One patient interviewed for a story was scheduled to undergo a total knee replacement procedure.
Unfortunately, according to a recent news article from the New York Times, things don’t always work out so well. One patient interviewed for a story was scheduled to undergo a total knee replacement procedure.
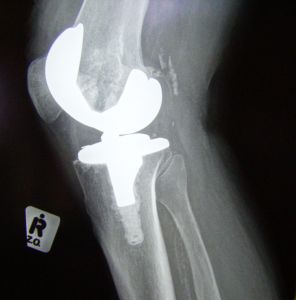
When a surgeon performs a total knee replacement, he or she relies on a medical device known as a cutting guide. Much like a carpenter’s jig, a surgical cutting guide is placed on the bone to be cut so the surgeon knows where to apply his or her bone saw. Cuts must be made in the precision location to increase the chances of a proper fit and alignment. If alignment is off, even slightly, patients can have a longer recovery, increased pain, decreased range of movement, and in worst cases, the artificial knee may fail and become loose or dislodged.
Continue reading
 Product Liability Lawyer Blog
Product Liability Lawyer Blog