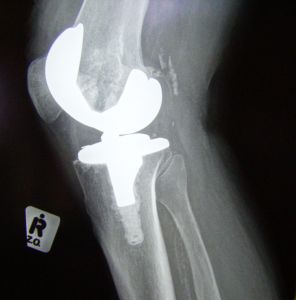
Knee replacement surgeries are on the rise, even in younger patients. New research suggests the number of knee replacements is parallel to the rising rate of obesity. Analysts report that the demand for the surgery is increasing, almost parallel with the rates of the overweight and obese in Boston and throughout the United States. According to the study, 95% of individuals who are in-line for or considering knee replacements have associated weight problems. This spike in knee replacement surgery for the obese and among young people has created the potential for defective replacements and the need for additional surgeries.
While doctors and experts may advise patients to seek knee replacements, patients should be aware of the potential risks associated with knee replacement products. Our Boston defective medical device attorneys are experienced in representing individuals who have suffered harm resulting from defective knee products. In addition to providing advocacy to our clients, we are committed to staying abreast of research, trends, and legal developments related to knee replacement products.
Continue reading
 Product Liability Lawyer Blog
Product Liability Lawyer Blog